Welcome to the GxP Cloud Compliance Summit
Make valuable connections with Drug Developers, Medical Device, CRO & CMO looking for long-term partners
Why Attend the GxP Cloud Compliance Summit?
Nothing is more important in the pharmaceutical and life sciences industries than patient safety and meeting regulations. This past year and the global disruption have highlighted how life sciences organizations need to respond to radical shifts through digitization. The 2nd GxP Cloud Compliance Summit will return to discuss the next steps following initial feasibility study and implementation – designed to help Pharmaceutical & Life Science Companies achieve GxP regulated security, privacy, transparency and compliance at any scale when migrating to the cloud.
With 18 trailblazing stories from AbbVie, Orchard Therapeutics, AWS, USDM, Chef/Progress, we will share practical takeaways from CFR21 part 11 to mapping out data migration to selecting the right cloud providers. Across 2 days, attendees will gain the tools to streamline processes, facilitate on-demand scalability, increase efficiency and reduce costs.
This is the only dedicated industry-led meeting focused on GxP cloud adoption in the life sciences industry, join us this July to learn how to overcome the barriers of GxP cloud compliance, increase knowledge for data-powered business and empower collaboration between your organization and platform partners – providing a much more efficient operation and effective infrastructure to support your research and clinical activities.
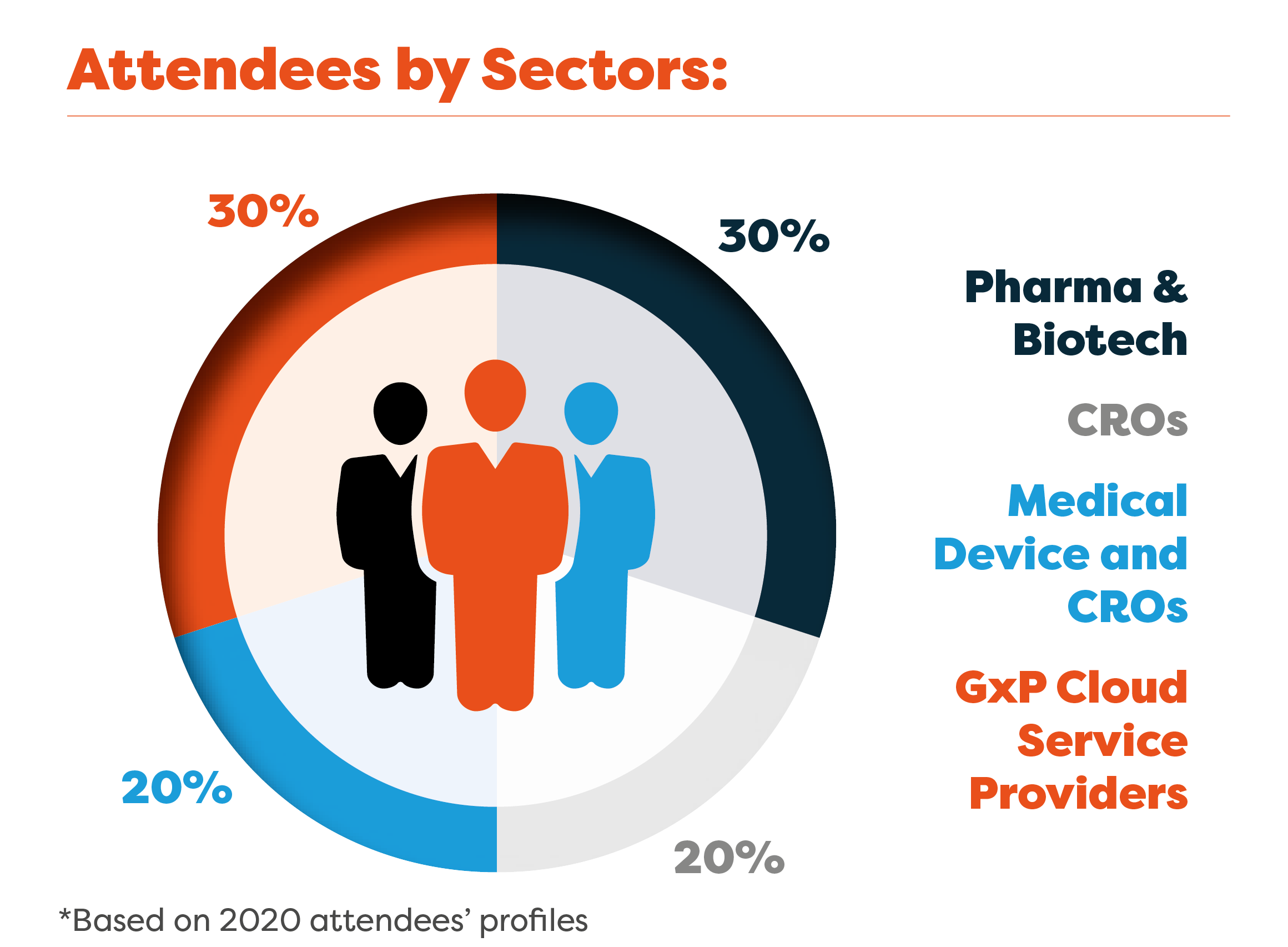
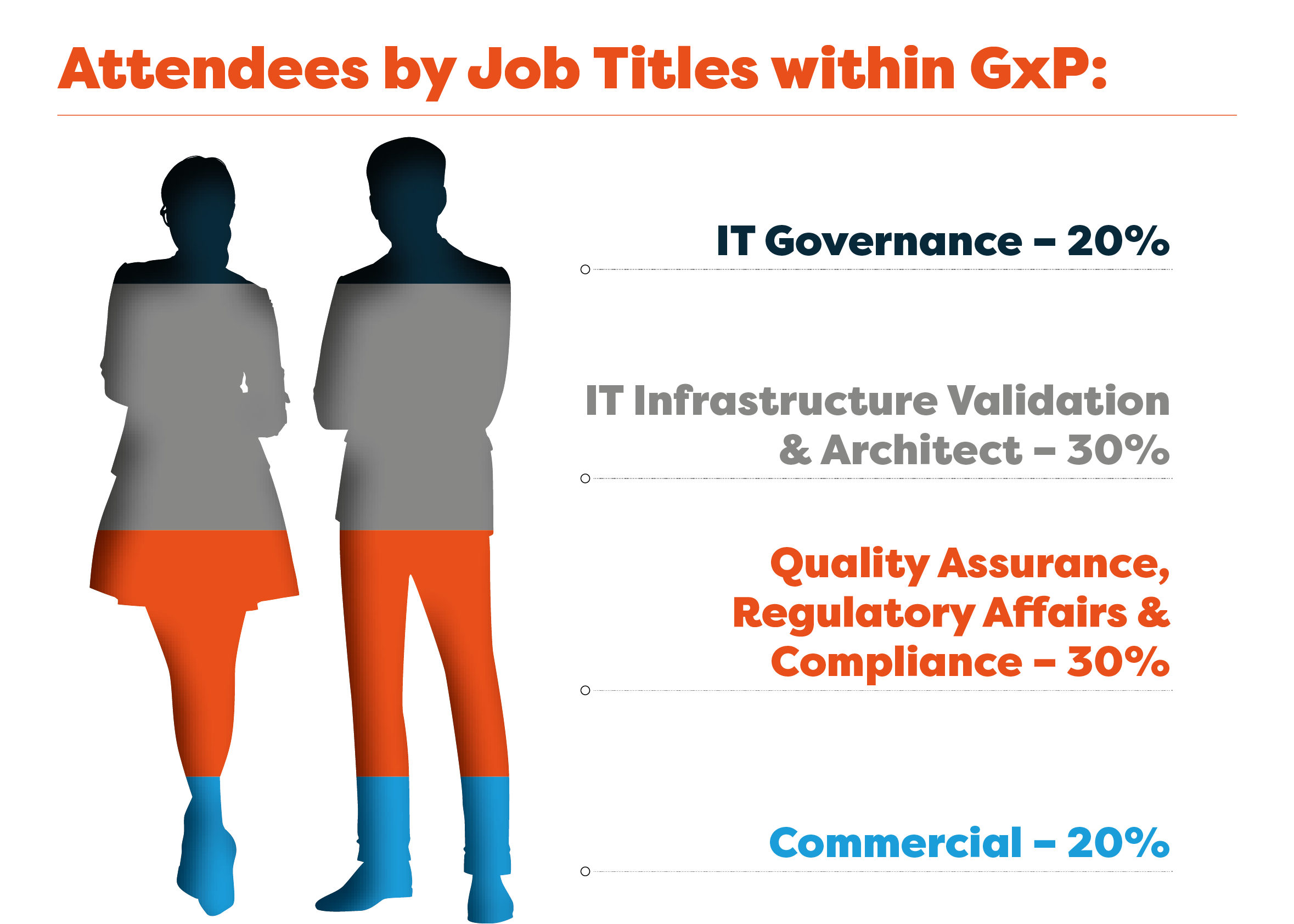
Who Our GxP Cloud Compliance Community Are Looking For:

Cloud and Platform Service Providers
Cloud Validators
Auditors and Consultants
Cloud Security Services
Companies You Will Meet:

Or if you would like to attend the event as a delegate; Secure a pass today:
Expertise Partners
Exhibition Partners
*Free Pass for Industry Practitioners: Drug Developers, Medical Device, CROs, CMOs & Research Institutions
Eligibility of free pass is subject to the organizer’s approval










